Ovule Sterility and Seed Set in Alfalfa
Daniele Rosellini12, Francesco Ferranti3 and Fabio Veronesil
' Istituto di Miglioramento Genetico Vegetale and 3 Dipartimento di Biologia Vegetale, Universita degli Studi di Perugia, Borgo XX giugno 74, 06100 Perugia, Italy. Research supported in part by the Italian Ministry of University and Scientific Research, Project: 'Characterization of mutations affecting sporogenesis and gametogenesis in Medicago spp.'. 2 Corresponding author, E-mail: roselli@krenet.it.
ABSTRACT
Seed potential is poorly realized in alfalfa seed production and seed set is generally low. Ovule sterility associated with callose deposition has been recently reported in alfalfa and could contribute to low seed-ovule ratio. Inheritance is sporophytic and narrow sense heritability is 85%. An R~LP mapping study demonstrates that a chromosome region explains a major portion of the variation for ovule sterility and also for cross- and self- fertility. The first results of cytological analyses show that no embryo sac develops in sterile ovules and that callose deposition begins just after the meiotic stage, affecting mainly the integumentary tapetum and nucellar cell walls. Nucellar cells expand to fill the space normally occupied by the embryo sac. In plants with 100% sterile ovules pistil growth often ceases at bud stage and a short, aborted pistil is found within the staminal colurnn at anthesis. Nine populations were analyzed and 4 to 26% sterile ovules per plant were found on average, with significant differences between populations and high variation within populations. Significant negative correlation was found between the percentage of sterile ovules and seeds per pod in most populations. Selection against ovule sterility should be easy, using a quick stain clearing technique based on callose fluorescence, and effective, due to high heritability. Checking ovule fertility in parental genotypes selected for variety development could help breeders obtain good seed yielding cultivars.
Ovule Sterility and Callose Deposition in Alfalfa
The realized seed potential is very low in alfalfa (Medicago sativa L., 2n=4x=32): it is estimated that seed/ovule ratio in the field is about 0.08 (Lorenzetti 1993). A major cause of this deficit is the low number of seeds produced per pod: of 10 ovules present in the floret, on average, usually no more than 5 become seeds. Removing at least some of the causes that limit seed potential would be beneficial, since little effort has been put in improving seed yield, and alfalfa can be considered still wild for seed traits. An ovule sterility trait associated with retarded integument development controlled by a single recessive gene has been described in alfalfa by Bingham and Hawkins-Pfeiffer (1984). In a recent study (Rosellini et al. 1998) a new genetic ovule sterility trait has been demonstrated in a plant from the alfalfa cultivar Blazer XL, named B17, which has 81% ovules displaying heavy callose deposition at the time of anthesis (Fig. 1) and low female fertility. Callose appears to surround the embryo sac site of 40- 100% ovules per floret at the time of anthesis (Tab. 1). Florets with 100% callosized ovules were frequent. Sterility of callosized ovules was demonstrated using six progeny plants obtained from B17 displaying 100% callosized ovules. These plants were female sterile when hand crossed with an unrelated male fertile plant. In some plants with very high percentage of sterile ovules, under-developed pistils were found at anthesis, half or less the size of normal ones, that did not emerge from the staminal column.
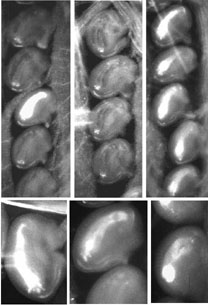 Fig. 1 Photo micrographs of cleared pistils showing callose deposition in the ovules of alfalfa plants. Ovaries are shown were 100%, one and no callosized ovules are seen; Different degrees and patterns of callose deposition can be seen in the lower images.
Florets were sampled at flowering and fixed in 70% ethanol:acetic acid 3:1 for at least 24 hours. The pistils were then dissected, cleared in 8N NaOH for 5 hours, stained overnight in 0.1% aniline blue in 0.1M K3PO4 (Martin 1959), mounted in the staining solution and gently squashed under a coverslip. Fluorescence microscopy was performed according to Barcaccia et al. (1996) (from Rosellini et al. 1998)
Fig. 1 Photo micrographs of cleared pistils showing callose deposition in the ovules of alfalfa plants. Ovaries are shown were 100%, one and no callosized ovules are seen; Different degrees and patterns of callose deposition can be seen in the lower images.
Florets were sampled at flowering and fixed in 70% ethanol:acetic acid 3:1 for at least 24 hours. The pistils were then dissected, cleared in 8N NaOH for 5 hours, stained overnight in 0.1% aniline blue in 0.1M K3PO4 (Martin 1959), mounted in the staining solution and gently squashed under a coverslip. Fluorescence microscopy was performed according to Barcaccia et al. (1996) (from Rosellini et al. 1998)
Cytological Evidences and Stability of Ovule Sterility
Among the Ft progeny obtained by crossing B17 by P13 (a plant from a Peruvian accession showing 5% callosized ovules) two plants contrasting for ovule-sterility percentage were selected: F,-48, which shows 98% callosized ovules and 42% under-developed pistils, and F~-22, which has 0.52% callosized ovules and no under-developed pistils. We fixed florets of these two plants in different developmental stages and we are currently examining semi-thin sections after toluidine blue and anilin blue staining. The first results have showed that functional megaspores can be seen in F,-22, while, at the same stage, a megaspore mother cell is found in F, 48. We are going to examine earlier stages of development to assess whether meiosis is regular or
not in F,-48. An hypothesis is that meiosis does not occur in Fl-48, and the megaspore mother cell is blocked in its development. In fact, callose is normally deposited around the megaspore mother cell and is degraded after non-fuctional megaspore degeneration (Barcaccia et al. 1996). It is possible that meiosis is blocked and callose is not degraded but deposited further, maybe to act as a molecular filter (Kapila and Tiwari 1978) preventing pollen tube-attracting substances from guiding pollen tubes to sterile ovules. In fact, pollen tubes were seen to bypass callosized ovules. At the functional megaspore stage, callose is absent in normal ovules but is already found in the walls of the cells surrounding the nucellus (integumentary tapetum) and inside the nucellus in Fl-48 ovules. While an embryo sac develops in Ft-22, in F,-48 nucellar cells appear to expand filling the space normally occupied by the embryo sac. The integumentary tapetum cells also are much bigger than in F,-22, and callose deposition continues in their walls. At anthesis, no embryo sac is found in F,-48. To assess the stability of ovule sterility, S plants were evaluated in subsequent regrowths (Tab. 1). Variation of percent ovule sterility among florets of a plant was relatively high but repeated sampling of the same plants over time showed that the expression of the trait was comparatively stable (Rosellini et al. 1998). Further evidence has been obtained using 50 ~7~13 F, progeny plants, whose sterilities in each of two regrowths were highly correlated (r=0.g6). In this progeny, the percentage of under-developed pistils was significantly correlated with sterility, as expected. Ovule sterility was not correlated with pollen production or plant vigor, b~t a significant correlation was found between sterility and flower color score (1, very pa~e purple, to 5, intense purple). Two plants with very pale flower color showed very high ovule sterility and there was an evident sterility decrease moving toward intense flower color. Linkage between sterility and flower color genes or physiological mechanisms could explain this result. The possible role of developing ovule nutriti~n in sterility was investigated by recording the percentage of callosized ovules in relation to the position of the ovules within the ovary for B 17 and other three plants (Fig.2). The ovules closer to the stigmatic end of the pistil showed the same sterility as the ones furthest from the stigma. This applies to highly sterile and highly fertile genotypes, and indicates that sterility should not depend on nutrient transport, as would be the case if, for example, callose was found more in distal than in proxirnal ovules.
Inheritance of Ovule Sterility.
Progenies of B17 were obtained and studied to investigate the inheritance of ovule sterility (Rosellini et al 1998). B17 transmitted ovule sterility to the progenies, and this is proof for sporophytic genetic control. Plants B17 and P13 produced clearly distinct S1 progenies whose means were very close to the parents. Reciprocal Fl and backcross progenies of B17 and P13 displayed continuous variation and the means were intermediate between the parents. This was true also for the Fl progenies of B17 by BP and 6-4 (plants with very low sterility, see Fig. 2). The regression of the mean ovule sterility of offspring on midparents provided an estimate of narrow-sense heritability of 0.85, with a standard error of 0.11, demonstrating that additive gene action is prevalent and that a small number of genes should be involved. Additional insight on genetic control is provided by unpublished data by D. J. Brouwer. In a RFLP mapping study conducted on about 100 plants obtained by backcrossing B17 with a F~ (B17xP13) plant characterized by very high sterility, Tab. 1) he found a chromosome region that explained a major portion of the variation for ovule sterility. Each of seven markers in this 46.3 cM region explained from 5 to 17 % of the variation for ovule sterility, demonstrating the presence of a major QTL. The same markers explained a major portion of the variation for cross and self-fertility (seeds per pollinated floret), assessed in different years and with different pollen sources. These results encourage research aimed at isolating this major gene.
Relevance of Genetic Ovule Sterility for Seed Set and Seed Production
Ovule sterility affected seed set in two progeny populations (Table 2). In the Fl B17xP13, the number of seeds per pod was negatively correlated with the percentage of sterile ovules. Also the pod - floret ratio was significantly negatively correlated with sterility, as could be expected in a population with high sterility. Self- and cross-fertility were evaluated in different years and with different pollen sources in B 1 7x(FI-s) backcross population as seeds per pollinated floret, and they were always negatively correlated with ovule sterility. That ovule sterility affected seed set in these progenies characterized by a high level of sterility was expected. What is more interesting to assess is how widespread it is in
populations/cultivars and whether it can affect their seed set and yield. To help answer these questions, ovule sterility of nine alfalfa populations of different origin was studied. Twenty five plants from each of four North American cultivars (Blazer XL, Columbia 2000, Magnum III, Wl 0-AC3), one Italian cultivar (Adriana) and four populations (Peruvian, a non-dormant germplasm, Casalina, an Italian cultivated ecotype, Wisfal, a tetraploid M. falcata population developed by E.T. Bingham, and a diploid M. coerulea population) were examined. Callosized ovules were found in all of the populations, from a minimum of 4% average sterility per plant in Wisfal to a Maximum of 26% in M. coerulea, and the differences between populations were significant. Among the cultivated materials, the Italian populations were more fertile than the North American and Peruvian ones. A few plants with as many as 70% sterile ovules were found in three populations, and plants with no sterile ovules were rare. The fourth North American cultivars were hand cross-pollinated with pollen from an unrelated male fertile plant, and the italian ecotype was open pollinated in the field. Negative correlation coefficients were obtained between ovule sterility and seeds per pod (Tab. 4), and they were significant for three out of four cultivars. The high correlation coefficient observed for W10 AC3 indicates that ovule sterility was more important among the factors limiting seed set than in the other populations. Seed set with open pollination showed a negative but non-significant correlation with ovule sterility, with a larger sample, a significant coefficient would have probably been obtained. Overall, thousand seed weight was weakly positively correlated with ovule sterility, but not within populations. The predicted number of seeds per pod, calculated by subtracting the average number of sterile ovules from the average number of ovules per floret per plant, showed correlation coefficients with actual seeds per pod higher than those of sterility percentage per se in the North American cultivars (data not shown). The predicted number of seed per pod could be a good selection tool for improving seed set. It is possible that the sterility associated to callose is not controlled by the same genes in all the populations and all the plants within a population. In fact, different sterility genes and mechanisms may result in callose deposition within the ovule. However, assuming that callose is a
marker of sterility, it could be used as a selection tool irrespective of the genes that are involved in the different populations.
Conclusions
Genetic ovule sterility has been reported in several species (Klucher et al. 1996; Pereira et al. 1997). However, unless an easily scorable marker is available, collecting data on ovule sterility is time consuming and it is impractical to examine many plants. By associating ovule sterility with callose deposition we were able to examine hundreds of plants. That ovule sterility is an important factor in determining low seed set has been indicated by several studies. In Epilobium obcordatum (Seavey and Carter 1996) an average of 39% ovules were sterile. In a Lotus corniculatus plant 10.5% sterile ovules were counted (Rim et al. 1990). Pasumarty et al. (1993) examined the ovules of 4 Trifolium repens cultivars and reported that 28-33% of the ovules per carpel were sterile. In a study conducted on a single white clover pl~nt, a minimum of 37% of the ovules were found to be sterile (Thomas and Pasumarty 1996). Thomas (1996) has proposed ovule sterility as an important component of low seed set in Trifolium repens. In alfalfa, ovule sterility associated with deposition of callose in the nucellus has been reported (Kolyasnikova 1985; Oryol et al. 1986; Dzyubenko and Vyshniakova 1995). Huyghe et al. (1998) reported 2-64% ovule sterility in 90 plants from 10 populations. So far we have demonstrated that genetically controlled, sporophytic, additive ovule sterility is present in alfalfa. We also have a picture of how widespread ovule sterility is in wild and cultivated alfalfa populations, and we have shown that seed set is likely to be negatively affected. On the basis of cytological evidence, the involvement of genes controlling the meiotic process is possible and will be investigated. Why genetic ovule sterility is maintained in alfalfa population is a matter of speculation. Alfalfa is known to have a high genetic load (Kimbeng, 1996) and a perennial, outcrossing species is likely to tolerate a degree of ovule sterility higher than that of an annual species (Wiens et al., 1987). However, selective advantage could be hypothesized for plants producing few seeds if these seeds are larger and have higher fitness than small seeds produced by highly fertile plants.
Further study of this trait is encouraged by the interest of isolating and studying the genes involved, that could control meiosis, and the possiblity of improving seed set in cultivated alfalfa by selecting against ovule sterility. Using callose as a marker should make selection easy, and because of high heritability it should be effective. Moreover, plants can be evaluated for seed potential even in a green house and without pollination. Checking ovule fertility in parental genotypes selected for variety development could help breeders obtain good seed yielding
variehes.
Acknowledgements
We wish to thank E.T. Bingham, University of Wisconsin-Madison for providing plant materials, greenhouse space and all his experience; D. J. Brouwer, University of California-Davis, for unpublished data on RFLP mapping, Prof. F. Lorenzetti, University of Perugia-Italy, for his valuable advise, and P. Taviani, University of Perugia-Italy, for helping in exan~ining part of the population rnaterials.
REFERENCES
Barcaccia, G., A. Mazzucato, M. Falcinelli, and F. Veronesi. 1996. Callose localization in cell walls during meiotic and apomeiotic megasporogenesis in diploid alfalfa. Caryologia 49:45-56.
Bawa, K.S., and D.P. Buckley. 1989. Seed:ovule ratios, selective seed abortion, and mating system in Leguminosae. In: Stirton CH, Zarucchi JL (eds) Advances in legume biology. Monogr. Syst. Bot. Missouri Bot. Gard. 29:243-262.
Bingham, E.T., and J. Hawkins-Pfeiffer. 1984. Female sterility in alfalfa due to a recessive trait retarding integment development. J. Hered. 75:231-233.
Dzyubenko, N.I., and M.A.Vyshniakova. 1995. Intraspecific variability of seed productivity character in alfalfa. Proc. Third International Herbage Seed Conference, Halle, Germany, June 23-28.
Huyghe, C., et al. 1998. Proc. North American Alfalfa Improvement Conference, Bozeman, MN, USA, Aug 2-6.
Kapil, R.N., and S.C. Tiwari. 1978. Plant embryological investigations and fluorescence microscopy: anassessmentofintegration. Intl. Rev. Cytol. 53:291-331.
Kimbeng, C.A. 1996. Genetic experiments in alfalfa (Medicago sativa L.): fertility, genetic load and Sl forage yield performance in original and improved two-allele autotetraploid populations of alfalfa. Ph. D. thesis, University of Wisonsin-Madison, 95p, DA# 96 13353.
Klucher, K.M., E. Chow, L. Reiser, and R.L. Fischer. 1996. The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is reated to the floral homeotic gene APETALA2. Plant Cell 8:137-153.
Kolyasnikova, N.L. 1985. Determining the fertility of alfalfa by means of fluorescence microscopy. Botanicheskie issledovaniya na Urale 25.
Lorenzetti, F. 1993. Achieving potential herbage seed yield in species of temperate regions. Proc. XVII International Grassland Congress 1621-1628.
Martin, F.W. 1959. Staining and observing pollen tubes in the style by means of fluorescence. StainTechnol. 34:125-128.
Oryol, L.I., L.N. Kostantinova, V.F. Ogorodnikova, and N.I. Dzyubenko. 1986. The fertility of lucerne ovules and methods for its evaluation. Sbornik Nauchnykh Trudov po Prikladnoi Botanike, Genetike i Selektsii 99:10-17.
Pereira, T.N.S., H. Ilarslan, and R.G. Palmer. 1997. Genetic and Cytological Analyses of three lethal ovule mutants in soybean (Glycine max; Leguminosae). Genome 40:273-285.
Pasumarty, S.V., T. Matsumura, S. Higuchi, and T. Yamada. 1993. Causes of low seed set in white clover (Trifolium repens L). Grass and Forage Science 48:79-83.
Rosellini, D., F. Lorenzetti, and E.T. Bingham. 1998. Quantitative ovule sterility in Medicago sativa. Theor. Appl. Genet. 97:1289-1295.
Seavey, S.R., and S.K. Carter. 1996. Ovule fates in Epilobium obcordatum (Onagraceae). Amer. J. Bot. 83:316-325.
Thomas, R.G. 1996. Limitations to seed set in white clover (Trifolium repens L.). I. Preliminary observations. J. Appl. Seed Prod. 14:59-66.
Thomas, R.G., and S.V. Pasumarty. 1996. Limitations to seed set in white clover (Trifolium repens L.). II. The influence of light intensity on floral development. J. Appl. Seed Prod. 14:67-72.
Tiwari, S.C. 1982. Callose in the walls of mature embryo sac of Torenia fournieri. Protoplasma 110:1-4.
Wiens, D., C.L. Calvin, C.A. Wilson, C.I. Davern, D. Frank, and S.R. Seavey. 1987. Reproductive success, spontaneous embryo abortion and genetic load in flowering pl~ts. Oecologia 71 :501-509.