
Mary K. Sledge and Joe H. Bouton
Department of Crop and Soil Sciences
University of Georgia, Athens, GA 30602 USA
The development of genetic maps for cultivated
crops allows for the use of molecular markers for "marker-assisted selection".
Genetic maps provide a direct means to determine the chromosomal location
of genes controlling agronomic traits, the number of genes involved in
controlling a particular trait, and the gene action. This information will
allow for the manipulation of plant genomes in order to assemble "packages"
of desirable gene combinations. It also allows for the incorporation and
monitoring of genes from sources not available through conventional breeding,
such as the Bt genes for insect resistance. With genetic maps, it is possible
to compare the genes controlling phenotypes in different populations, as
well as across taxa. Molecular maps are also the first step in map-based
cloning of genes, which will allow the study of gene function, as well
as provide new opportunities for genetic engineering (Paterson, 1996).
Due to the complexity of tetrasomic inheritance, genetic maps of alfalfa have been constructed in diploid species of the Medicago sativa complex. Recently, mapping in tetraploids has become possible by mapping only alleles that are present in single dose (single-dose restriction fragments, or SDRFs) (Wu et al., 1992). Used in conjunction with diploid maps, SDRFs should provide a valuable tool for identifying and tagging traits of agronomic interest in cultivated alfalfa. Diploid maps have an important place in alfalfa improvement. The simplicity of diploid mapping allows any segregating marker to be mapped, not only those which occur in a single dose. This ability to map large numbers of markers makes possible the construction of high-density molecular marker maps which can be utilized for QTL identification and map-based cloning.
The usefulness of diploid maps in the breeding of tetraploid alfalfa depends on a high degree of similarity between the genomes of diploid and tetraploid alfalfa. If the same genes occur in diploid and tetraploid alfalfa, and in the same order, then diploid genetic maps can be used to identify important traits in tetraploid alfalfa. A high degree of genome conservation between diploid and tetraploid alfalfa is probable, based on 1) the high degree of genome conservation, or synteny, seen among members of numerous related species, 2) by chromosome pairing studies between members of the Medicago sativa complex, and 3) by the ability of diploid and tetraploid species to interbreed via 2n gametes.
Diploid genetic maps in alfalfa should
serve as an important framework for mapping in annual Medicago species,
as well as in tetraploid species, and for transferring unique genes identified
in diploids to the cultivated, tetraploid level. The development of a single,
highly saturated, composite diploid alfalfa map would provide an invaluable
source of genetic markers of known location. Since not all markers are
expected to segregate in all crosses, the more markers there are on the
map, the better the chances are of finding segregating markers in specific
crosses, and in specific chromosomal locations. A general strategy for
tagging traits in either annual medics, or in tetraploids, would be to
take evenly spaced markers from the diploid map, and look for segregation
of these markers in a specific cross. Once segregating markers are identified,
marker by marker methods, such as analysis of variance (ANOVA) could be
used to associate markers with the trait of interest. Once the trait is
localized to certain linkage groups, markers from these linkage groups
can be used to more precisely locate the trait (Figure 1). Mapping the
entire genome is not necessary, only the areas of interest.
 |
| Figure 1. Strategy for tagging traits with molecular markers. A) Select evenly spaced markers from current molecular map. B) Map these markers, along with trait of interest, in a population segregating for the trait of interest. C) Identify a significant marker by ANOVA. D) Map as many markers as possible in the region of the significant marker. Re-analyze for markers of significance. |
Genetic Mapping in Medicago
The autotetraploid nature of alfalfa makes mapping in the cultivated species difficult for two reasons: (1) A large number of genotypes is expected in a segregating population, and (2) due to comigration of fragments on agarose gels, identification of all genotypes is not possible (Wu et al., 1992). In a diploid, there are up to two alleles, and three possible genotypes per locus; in an autotetraploid, there are up to four alleles and nineteen possible genotypes per locus. Agarose gels can separate different alleles, but determining genotypes with multiple dose alleles would require the identification of doses of alleles. It would be difficult, for example, to determine from a blot of an agarose gel, the difference between an Aaaa genotype, and an AAAa genotype, as both would yield the same two bands. For these reasons, mapping of polyploids is often carried out in diploids. Identification of the three possible genotypes in a segregating diploid is straightforward. Autotetraploid species for which diploid genetic maps have been made include potato and alfalfa. Allotetraploid crops such as wheat are also mapped in diploids.
Wu et al. (1992) have proposed a method for mapping in polyploids that involves the use of SDRFs. SDRFs segregate in a 1:1 ratio, resembling the mapping of a diploid backcross population. Linkage groups must be constructed for each homologue of a chromosome, and then aligned based on the presence of SDRFs generated by the same DNA probe, or by mapping higher dosage markers. This method of mapping has been used in sugarcane, and more recently in alfalfa (Brouwer and Osborn, 1999). While it is difficult to generate enough markers to construct an entire map using this method, SDRFs should be useful in mapping specific regions of the genome associated with traits of interest.
Five genetic maps of alfalfa have been
published, four of which utilize diploid germplasm (Brummer et al., 1993,
2000; Kiss et al., 1993; Echt et al., 1994; and Tavoletti et al., 1996)
and one which utilizes tetraploid germplasm (Brouwer and Osborn, 1999).
Brummer et al. have mapped 130 distinct RFLP loci in 86 F2 plants from
a cross between Medicagosativa ssp. sativa (W2xiso (CADL))
and Medicago sativa ssp. coerulea (PI440501). In addition,
Diwan et al. (1999) added 9 SSR loci to this map. The average marker density
is 5.57 cM and total map length is 646.5 cM.
Echt et al. (1994) have constructed a diploid
map from a cross of two CADL parents. This map, which represents the genome
of cultivated, tetraploid alfalfa, reduced to the diploid level, was constructed
in a backcross population and consists of a map for each parent, joined
by common markers. Kiss et al. (1993) constructed a map from a cross between
Medicagosativa
ssp. quasifalcata and M. sativa ssp.
coerulea.
This map has 89 markers, including RFLP, RAPD, isozyme, and morphological
markers, and was made with an F2 population. The most recently published
diploid map is Tavoletti et al. (1996). This map was made from a cross
between a Medicago sativa ssp. falcata 2n egg mutant
and a CADL parent, and differs from preceding maps in that it is a non-inbred
map, constructed in an F1 population. Two maps were made, one for each
parent, and were joined by common RFLP markers. In addition to these published
diploid maps, Kiss et al. (1998) reports a map constructed from 137 F2
plants which has 868 markers, including 4 morphological markers, 12 isozyme
markers, 27 seed proteins, 213 RFLPs, 608 RAPDs, and 4 specific PCR sites.
These markers include 80 known genes.
Brouwer and Osborn (1999) constructed the
first tetraploid alfalfa map, using two backcross populations from a cross
between a non-dormant, winter-sensitive genotype from the Peruvian PI 536535
and a dormant, winter-hardy cultivar, Blazer XL. The map will be used to
identify QTL for dormancy, winter survival, and freezing tolerance.
A common feature of the inbred alfalfa maps is a high degree of segregation distortion. Segregation ratios for 18 to 50% of the markers deviated from the expected ratios, and tended to favor heterozygous genotypes. The most obvious explanation for this is that inbreeding uncovered deleterious recessive alleles (Osborn et al. 1998). The heterozygous loci tended to cluster together in linkage groups, and it is possible that not all linkages are valid. The non-inbred map constructed by Tavoletti et al. (1996) in an F1 population had only 8.8% segregation distortion. The use of non-inbred populations then, could be used to overcome this problem of segregation distortion. Kiss et al. (1998) have suggested another method to overcome problems associated with segregation distortion, called colormapping. Colormapping is a non-mathematical method for genetic mapping that converts numerical genotype scores to colors. These colored, graphical genotypes can be used to detect inaccuracies in mapping. Finally, the tetraploid map constructed by Brouwer and Osborn (1999) had only 4-9% segregation distortion, due to tetrasomic segregation and the low recovery of homozygous recessive individuals.
Mapping is also underway in another diploid
Medicago
species, Medicago truncatula (Huguet et al. 1997). M. truncatula
is an annual, self-fertile species which has been proposed as a model organism
for the study of Rhizobium-legume symbiosis. Several aspects of
M.
truncatula make it suitable as a model species, including small genome
size, short life cycle, and its capacity for rapid transformation and regeneration
(Nam et al., 1999). Information and tools developed during the study of
M.
truncatula could prove to be useful in the further development and
use of molecular maps in alfalfa. Covitz et al. (1998) developed a collection
of expressed sequence tags (ESTs) from a M. truncatula root-tip
cDNA library. A database of 899 sequences, 609 which show similarity to
known genes, is available via the World Wide Web. In addition to this,
Nam et al. (1999) developed a bacterial artificial chromosome (BAC) library
of M. truncatula
which can be utilized for map-based cloning and
physical mapping.
Genomic Similarity of Diploid and Tetraploid Alfalfa
Diploid and tetraploid alfalfa exhibit a large number of morphological and physiological differences, yet the genomes of these two forms are highly similar. Evidence supporting this includes 1) the high degree of conserved gene order, or synteny, between numerous related plant species, 2) the breeding behavior of diploid and tetraploid alfalfa, and 3) cytogenetic studies. Conservation of genes and gene order is expected between diploid and tetraploid alfalfa. Therefore, diploid genetic maps of alfalfa have direct application in the mapping and breeding of tetraploid alfalfa.
Synteny
Recent comparative mapping studies have
shown extensive colinearity, or synteny among the genomes of related, even
quite distantly related, species (Devos and Gale, 1997). The genomes of
two plant species are said to be in synteny when the genes, and the order
in which they occur on chromosomes, have been conserved throughout evolution.
Examples of this occur in both monocots and dicots, and include the following:
tomato and potato, Brassica species and Arabidopsis, numerous species
of grasses, rosaceous fruit trees, pines, and several legumes (Gale and
Devos, 1998). Conservation of gene order within plant families appears
to be the rule, rather than the exception (Gale and Devos, 1998). For example,
despite a large difference is genome size, extensive colinearity is seen
between rice and maize. These species diverged more than 50 million years
ago, and differ in both the basic chromosome number and the haploid DNA
content. Nevertheless, 85% of rice clones tested cross-hybridized to maize,
and 72% of rice sequences were actually duplicated in maize (Dean et al.,
1995). Given the close relationship between diploid and tetraploid alfalfa,
it is highly probable that the two genomes are colinear, with highly conserved
gene orders. Therefore, it is also probable that gene order in tetraploid
alfalfa can be inferred from the gene order in diploid alfalfa.
Breeding Behavior of Diploid and Tetraploid Alfalfa
Evidence that supports conservation of
the diploid and tetraploid alfalfa genomes is the fact that they interbreed
freely, and are considered to be subspecies of one another. The genus Medicago
has several diploid and tetraploid forms, including the tetraploid Medicago
sativa ssp. sativa, which is the cultivated species. Other forms
are diploid M. sativa ssp. coerulea, M. sativa ssp.
falcata, which has both diploid and tetraploid forms, and tetraploid
M.
sativa ssp. glutinosa. Together these are referred to as the
Medicago
sativa complex. The only barrier to interbreeding among the subspecies
is ploidy level, and this is often broken by the production of tetraploids
from diploids producing 2n gametes (Quiros and Bauchan, 1988). Diploid
germplasm has been used in the development of several tetraploid alfalfa
cultivars. The diploid PI 20725, M. falcata, was an important component
of Rhizoma and Narragansett alfalfa. Subsequent cultivar development utilizing
these cultivars led to the presence of PI 20725 in the pedigrees of more
than thirty cultivars (Rumbaugh, 1991).
Cytogenetic Similarity of Diploid and Tetraploid Alfalfa
Numerous chromosome pairing studies have
demonstrated a high degree of chromosome similarity among members of the
Medicago
sativa complex. Hybrids between these subspecies show excellent chromosome
pairing, and have no hybrid sterility in the F1 or later generations (McCoy
and Bingham, 1988). More recently, fluorescent in situ hybridization
has been used to compare the nucleolus organizing regions (NORs) of tetraploid
M.
sativa ssp. sativa, and diploid M. sativa ssp.
falcata
and M. sativa ssp. coerulea (Calderini et al. 1996). The
NORs occur on the constricted regions of satellited chromosomes only, in
both diploids and tetraploids. Calderini et al. (1996) conclude that if
polyploidization of M. sativa occurred via 2n gametes involving
either M. sativa ssp. falcata, or M.
sativa
ssp. coerulea, then little reorganization of rDNA loci has occurred
since that time. This is further support for the colinearity of these two
genomes.
Differences Between Diploid and Tetraploid Alfalfa
If the genomes of diploid and tetraploid
alfalfa are so similar, then differences seen between the two ploidy levels
must be related to the difference in chromosome number itself. Diploid
and tetraploid alfalfa differ for a great number of characteristics. These
have been reviewed by Bingham et al., 1994, and include leaf size, vigor,
forage yield, stress resistance, nitrogen fixation, expression of early
maturity, physiological differences, differences in breeding behavior,
and manifestation of heterosis. A possible explanation for these differences
is complementary gene interactions.
Complementary gene interactions are interactions
between dominant alleles at loci linked in repulsion. There are more complementary
interactions possible in autotetraploids than in diploids (Bingham et al.,
1994). Two factors contribute to this difference. Tetrasomic segregation
produces a higher number of heterozygous individuals than disomic segregation.
Disomic segregation in diploids produces homozygous dominant, heterozygous,
and homozygous recessive individuals in a ratio of 1:2:1. The corresponding
ratio for tetrasomic segregation is 1:34:1. The greater number of heterozygous
individuals produced by tetraploids gives them the potential to have a
higher frequency of loci containing at least one favorable, dominant allele.
The second factor is that dominant allelic states exist in tetraploids
that are not possible in diploids. In a diploid cross, for a linkat with
four loci, two of which are heterozygous in one parent, and the other two
heterozygous in the other parent, the progeny obtained can have no more
than two dominant alleles at the linkat. The same cross in an autotetraploid
can produce two, three, or four dominant alleles at the linkat. This greater
number of complementary interactions in the autotetraploid, rather than
actual differences in genes or gene order, could explain the differences
seen between diploid and tetraploid alfalfa.
Application of Diploid Maps
DNA markers and genetic maps have many
applications in plant breeding. Strain identification for plant variety
protection, assessment of genetic diversity, accelerated introgression
or backcross conversion, and the mapping of complex traits are some key
areas (Paterson, 1996). Diploid maps of Medicago sativa can be used
in this way for: (1) breeding of annual, diploid Medicago species
(2) mapping and breeding of cultivated, tetraploid alfalfa, and (3) facilitating
gene transfer between ploidy levels.
Breeding of Diploid Species
Annual species of Medicago, or annual medics,
have been grown extensively for winter forage and green manure in Mediterranean
regions, but have only recently been evaluated for use in North America
(Zhu et al., 1996). They are fast growing, produce a large amount of biomass,
and bear many seed pods, with hard seeds that remain viable in the soil
for long periods (Mariani et al., 1996). Annual medics have recently been
evaluated for weed control by intercropping with barley and corn (DeHaan
et al., 1997), for control of soil erosion and to provide nitrogen to subsequent
crops (Moynihan et al., 1996), and for use as an emergency forage in northern
locations experiencing winter-kill of alfalfa (Shrestha et al, 1998). These
studies all conclude that annual medics have potential in these areas,
but that further studies are needed to identify more desirable genotypes.
Current diploid maps of alfalfa could be
useful in the identification and breeding of superior annual medic genotypes.
Molecular markers derived from diploid M. sativa have already
been shown to be useful in the study of annual medics. Mariani et al. (1996)
have shown that RFLP markers derived from an alfalfa PstI library could
hybridize to 31 accessions from 13 annual medic species. Diwan et al. (1997)
have shown that 4 SSR markers are able to be amplified in tetraploid, diploid,
and annual Medicago species. Therefore, it is possible to apply
markers from perennial, diploid M. sativa maps to annual Medicago
species, not only for phylogenetic studies, but also for map construction,
or for inferring location of significant markers from the diploid M.
sativa map by colinearity.
Tetraploid Mapping and Breeding
Diploid maps of Medicago sativa also have application in the mapping of tetraploids. Diploid maps can serve as a source of markers, and can provide information about linkage order. Li et al. (1998) used common AFLP markers to locate the R2 allele, conferring resistance to Phytophthora infestans in potato (Solanum tuberosum). Eleven AFLP markers cosegregating with the resistance gene were identified by bulked segregant analysis. These markers were then mapped into a single linkage group. Three of the markers could be located, in the same order, on a diploid map of a reference mapping population. The position of the R2 allele was inferred from the position of the three common markers.
Ploidy Level Gene Transfer
Gene transfer between ploidy levels via
2n gametes is commonly used in potato breeding. The cultivated potato is
an autotetraploid, vegetatively propagated species with high levels of
heterozygosity (Carputo et al., 1997). There are about 200 wild, tuber
bearing species of potato, 70% of which are diploid. They have high levels
of heterozygosity as measured with RFLP markers, and carry many resistance
genes to biotic and abiotic stresses common in potato crop production (Watanabe
et al., 1994). In contrast, cultivated potato has a narrow genetic base,
and sources of resistance to common pathogens cannot be found in cultivated,
tetraploid germplasm. In order to take advantage of the genetic variation
found in diploid germplasm, a systematic approach has been developed, taking
advantage of the production of 2n gametes. First, haploids are extracted
from tetraploid cultivars. These haploids are then crossed with wild diploids,
or to reduce the amount of wild germplasm, with hybrids of haploids and
wild diploids. The haploid-species hybrid, which produces 2n gametes, is
then crossed to tetraploid S. tuberosum, and tetraploid progeny
are recovered (Figure 2). In this way, valuable genes from wild genetic
resources are transferred from the diploid to the tetraploid level, and
introgressed into cultivated potato (Watanbe et al., 1994).
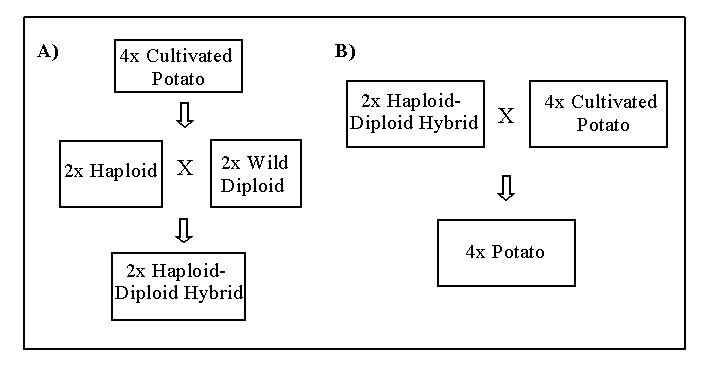 |
| Figure 2. Ploidy level gene transfer in potato. A) Haploids are extracted from cultivated potato, and crossed with wild diploids. Haploid-diploid hybrids are recovered. B) Haploid-diploid hybrids carrying the trait of interest and producing 2n gametes are crossed back to cultivated potato and tetraploid progeny carrying the introgressed trait are recovered. |
This method has been used to introgress
quantitative resistance to bacterial wilt, early blight, potato tuber moth
and root-knot nematode (Watanabe et al. 1995). Molecular markers can assist
with gene transfers across ploidy levels. Currently, molecular marker information
is being employed to facilitate genotypic selection in potato breeding,
rather than relying on phenotypic measurements. Joint efforts are underway
to simultaneously select QTLs for glandular trichomes, tuberization traits,
and tuber dormancy. In addition, molecular markers have been used to accelerate
selection for resistance to PVX, a single, dominant inherited trait (Watanabe,
1995).
Ploidy level gene transfers via 2n gametes
are also possible in alfalfa, and could be used to transfer valuable traits
from diploid relatives into cultivated alfalfa. A few examples of ploidy
level transfers are reported. Kimbeng et al. (1997) transferred a black
seed trait, conditioned by at least three genes, from the diploid to the
tetraploid level via 2n eggs produced by the diploid. Endre et al. (1996)
reduced a tetraploid, non-nodulating alfalfa from the tetraploid to the
diploid level, in order to more easily map the two recessive mutations
responsible for the trait. In both cases, the complexity of tetrasomic
inheritance was avoided by making use of ploidy level manipulations. This
is especially important in the case of recessive genes, which would require
the use of extremely large tetraploid populations in order to recover progeny
expressing the recessive gene. Finally, quantitative trait loci (QTL) controlling
aluminum tolerance have been identified in diploid M. sativa ssp.
coerulea (Sledge et al., 1997), and efforts are currently underway
to transfer this trait to cultivated alfalfa via 2n gametes.
Conclusions
The development of genetic maps for cultivated
crops opens new avenues for plant breeding. In alfalfa, map construction
has been carried out at the diploid level, in order to avoid the complexities
of mapping at the tetraploid level. Diploid alfalfa differs from tetraploid
alfalfa for many agronomic traits. These differences are probably due to
the greater number of complementary gene interactions possible in tetraploids
than in diploids, not to differences of genes or gene order. Diploid and
tetraploid forms of alfalfa are cytologically very similar, and are able
to interbreed due to the production of 2n gametes. Because of this similarity,
they are considered to be subspecies, and are collectively referred to
as the
Medicago sativa complex. Given this similarity, along
with the colinearity of the genomes of both monocot and dicot species,
as revealed by comparative mapping, it is likely that genomes of diploid
and tetraploid alfalfa are highly colinear. Conserved marker orders will
make diploid alfalfa maps useful for the breeding of annual Medicago
species, for identification and transfer of genes across ploidy levels,
and for the mapping and breeding of tetraploid alfalfa.
References
Bingham, E.T., R.W. Groose, D.R. Woodfield, and K.K. Kidwell. 1994. Complementary gene interactions in alfalfa are greater in autotetraploids than diploids. Crop Sci. 34:823-829.
Brouwer , D.J., and T.C. Osborn. 1999. A molecular marker linkage map of tetraploid alfalfa (Medicago sativa L.). Theor. Appl. Genet. In Press.
Brummer, E.C., J.H. Bouton, and G. Kochert. 1993. Development of an RFLP map in diploid alfalfa. Theor. Appl. Genet. 86: 329-332.
Calderini, O., F. Pupilli, P.D. Cluster, A. Mariani, and S. Arcioni. 1996. Cytological studies of the nucleolus organizing regions in the Medicago complex: sativa-coerulea-falcata. Genome 39:914-920
Carputo , D., A. Barone, T. Cardi, A. Sebastiano, L. Frusciante, S.J. Peloquin. 1997. Endosperm balance number manipulation for direct in vivo germplasm introgression to potato from a sexually isolated relative (Solanum commersonii Dun.). Proceedings of the National Academy of Sciences of the United States of America. 94(22):12013-12017.
Covitz, P.A. L.S. Smith, and S.R. Long. 1998. Expressed sequence tags from a root-hair-enriched Medicago truncatula cDNA library. Plant Physiol. 117:1325-1332.
Dean, C., and R. Schmidt. 1995. Plant genomes: a current molecular description. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46:395-418.
De Haan, R.L., C.C. Sheaffer, and D.K. Barnes. 1997. Effect of annual medic smother plants on weed control and yield in corn. Agron. J. 89:813-821.
Devos, K.M., and M.D. Gale. 1997. Comparative genetics in the grasses. Plant Mol. Biol. 35:3-15.
Diwan, N., A.A. Bhagwat, G.B. Bauchan, and P.B. Cregan. 1997. Simple sequence repeat DNA markers in alfalfa and perennial and annual Medicago species. Genome. 40:887-895.
Diwan, N., J.H. Bouton, G. Kochert, and P.B. Cregan. 1999. Mapping simple sequence repeats (SSR) DNA markers in diploid and tetraploid alfalfa. Theor. Appl. Genet. [in press].
Echt, C.S., K.K. Kidwell, S.J. Knapp, T.C. Osborn, and M.J. McCoy. 1994. Linkage mapping in diploid alfalfa (Medicago sativa). Genome. 37:61-71.
Endre, G., P. Kalo, M. Hangyel Tarczy, C. Csanadi, and G.B. Kiss. 1996. Reducing the tetraploid non-nodulating alfalfa (Medicago sativa) MnNC-1008(NN) germ plasm to the diploid level. Theor. Appl. Genet. 93:1061-1065.
Gale, M.D. and K.M. Devos. 1998. Plant comparative genetics after 10 years. Science 282:656-658.
Huguet, T., L. Tirichine, M. Gherardi, M. Sagan, G. Duc, J. -M. Prosperi. 1997. Molecular genetics of a model-plant: Medicago truncatula. p.259-262. In A. Legocki, H. Bothe, and a. Puhler (eds.) Biological fixation of nitrogen for ecology and sustainable agriculture. Springer-Verlag, Berlin.
Kidwell, K.K., D.R. Woodfield, E.T. Bingham, and T.C. Osborn. 1994. Molecular marker diversity and yield of isogenic 2x and 4x single-crosses of alfalfa. Crop Sci. 34:784-788.
Kimbeng, C.A., and E.T. Bingham. 1997. Backcrossing a complex black seed trait from diploid into tetraploid alfalfa avoids the complexities of tetrasomic inheritance. Crop Sci. 37:1376-1378.
Kiss, G.B., G. Csanadi, K. Kalman, P. Kalo, and L.O. Kresz. 1993. Construction of a basic genetic map for alfalfa using RFLP, RAPD, isozyme, and morphological markers. Mol. Gen. Genet. 238:129-137.
Kiss, G.B., P.Kalo, P.Kiss, K. Felfoldi, A. Kereszt, and G. Endre. 1998. Construction of an improved genetic map of diploid alfalfa (Medicago sativa) using a novel linkage analysis for chromossoal rregions exhibiting extreme distorted segregation. p. 313. In C. Elmerich, A. Kondorosi, and N.E. Newton (eds.) Biological nitrogen fixation for the 21rst century. Kluwer Academic Publishers, Dordrecht.
Kiss, G.B., A. Kereszt, P. Kiss, and G. Endre. 1988. Colormapping: A non-mathematical procedure for genetic mapping. Acta Biologica Hungarica. 49:125-142.
Li.,X., H.J. van Eck, J.N.A.M. Rouppe van der Voort, D.-J. Huigen, P. Stam, and E. Jacobsen. 1998. Autotetraploids and genetic mapping using common AFLP markers: the R2 allele conferring resistance to Phytophthora infestans mapped on potato chromosome 4. Theor. Appl. Genet. 96:1121-1128.
Mariani, A., F. Pupilli, and O. Calderini. 1996. Cytological and molecular analysis of annual species of the genus Medicago. Can. J. Bot. 74:299-307.
McCoy, T.J., and E.T. Bingham. 1988. Cytology and cytogenetics of alfalfa. p.737-776. In A.A. Hanson, D.K. Barnes, and R.R. Hill (eds.) Alfalfa and alfalfa improvement. American Society of Agronomy, Madison.
Moynihan, J.M., S.R. Simmons, and C.C. Scheaffer. 1996. Intercropping annual medic with conventional height and semidwarf barley grown for grain. Agron. J. 88:823-828.
Nam, Y.-W. , R.V. Penmetsa, G. Endre, P.Uribe, D. Kim, and D.R. Cook. 1999. Construction of a bacterial artificial chromosome library of Medicago truncatula and identification of clones containing ethylene-response genes. Theor. Appl. Genet. 98:638-646.
Osborn, T.C., D.J. Brouwer, K.K. Kidwell, S. Tavoletti, and E.T. Bingham. 1998. Molecular marker applications to genetics and breeding of alfalfa. In: E. C. Brummer, N.S. Hill, and C.A. Roberts (eds.) Molecular and cellular technologies for forage improvement. CSSA Sspecial Publication Number 26, Crop Science Society of America, Inc., Madison.
Paterson, A.H. 1996. Mapping genes responsible for differences in phenotype. p.41-54. In A.H. Paterson (ed.) Genome mapping in plants. R.G. Landes Company.
Paterson, A.H. 1996. DNA marker-assisted crop improvement. p.41-54. In A.H. Paterson (ed.) Genome mapping in plants. R.G. Landes Company.
Quiros, C.F. and G.R. Bauchan. 1988. The genus Medicago and the origin of the Medicago sativa complex. p. 93-124. In A.A. Hanson, D.K. Barnes, and R.R. Hill (eds.) Alfalfa and alfalfa improvement. American Society of Agronomy, Madison.
Rumbaugh, M.D. 1991. Plant introductions: The foundation of North American forage legume cultivar development. In: H.L. Shands and L.E. Wiesner (eds.) Use of plant introductions in cultivar development. Part 1. CSSA Special Publication Number 17, Crop Science Society of America, Inc., Madison.
Shrestha, A., O.B. Hesterman, J.M. Squire, J.W. Fisk , and C.C. Sheaffer. 1998. Annual medics and berseem clover as emergency forages. Agron. J. 90:197-201.
Sledge, M.K., J.H. Bouton, J. Tamulonis, W.A. Parrott, and G. Kochert. 1997. Aluminum tolerance QTL in diploid alfalfa. Proc XVIII Int. Grassland Congr. Winnipeg, Canada, 8-19 June.
Tavoletti, S., F. Veronesi, and T.C. Osborn. 1996. RFLP linkage map of an alfalfa meiotic mutant based on an F1 population. J. Hered. 87:167-170.
Watanabe, K., M. Orillo, M. Iwanaga, R. Ortiz, R. Freyre, and S. Perez. 1994. Diploid potato germplasm derived from wild and landrace genetic resources. Am. Pot. J. 71:599-604.
Watanabe, K.N., M. Orillo, and A.M. Golmirzaie. 1995. Potato germplasm enhancement for resistance to biotic stresses at CIP-Conventional and biotechnology-assisted approaches using a wide-range of solanum species. Euphytica. 85:45i7-464.
Wu, K.K., W. Burnquist, M.E. Sorrells, T.L. Tew, P.H. Moore, and S.D. Tanksley. 1992. The detection and estimation of linkage in polyploids using single-dose restriction fragments. Theor. Appl. Gen. 83:294-300.
Zhu, Y., C.C. Sheaffer, and D.K. Barnes. 1996. Forage yield and quality of six annual Medicago species in the north-central USA. Agron. J. 88:955-960.